You are currently browsing the category archive for the ‘Engagement’ category.
Twelve hours after arriving in Munich, I found myself in a beautiful tree-lined side street of the city at a diabetes bloggers event coordinated by Roche Diabetes Care. Fuelled by nothing more than coffee and jet lag, I walked into a beautiful building and found myself surrounded by diabetes advocates from around Europe who were probably trying to work out why an Australian had crashed their meeting.

Bastian takes the stage.
Firstly, a little about this group. Roche convened the blogger group a few years ago as a channel to build a relationship with PWD in Europe. (Roche has had a long history of working with consumers. I remember back in 2012 watching the Roche Diabetes Summit in awe and then trying to replicate it here with Australia’s first and only SoMe Summit.) In a very smart move, they engaged DEDOC leader and nice-guy extraordinaire Bastian Hauck to be the liaison between Roche and the community. Bastian has done a stellar job bringing together some absolutely amazing and influential advocates to be part of this work.
The group has now met a few times, and at this year’s EASD, they opened the door to an Australian (slightly less weird now that Australia is part of Eurovision, which, obviously, is the new gold standard measure of inclusiveness. First Eurovision digression.)
The first part of the afternoon session was a demonstration of the yet-to-be-released Roche CGM. A short presentation showed how the device works, with an explanation of the technology. The timeline for release of the product is later this year with launch markets being Sweden, Norway, Netherlands and Denmark.
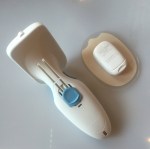
The soon-to-be-released Roche Insight CGM system.
We were then able to have a play with the device, inserting sensors into ‘fake’ skin pads and just getting an idea of the feel, size and look of it. The CGM app is completely customisable. It looks great – super clean and easy to use.
In a room of generally tech-savvy folk, you can imagine that there were a lot of opinions and feedback about the device. Most, if not all, of the participants were wearing at least one medical device – whether that be a pump, CGM or flash GM (and the slightly OTT Aussie who was wearing all three). We are obviously not the norm, but given our knowledge and experience with diabetes tech, we certainly did have a lot to say.

Dexcom and Insight side-by-side comparison.
There were some things that people really liked about the product. Accuracy was outstanding with MARD being comparable to Dex G5. The profile of the sensor was good – about the same as – maybe slightly lower than – the G5 on my arm when compared side by side. Insertion was super-easy and definitely doable with one hand. And the tape holding the sensor in tape is, apparently, better for people with skin allergies.
But as a first generation CGM, there were some limitations that people felt would frustrate them. The lack of integration with the Roche pump, for example, was of concern – however, this will be remedied with future generations. The first gen will only be compatible with an HTC phone (in a room full of very pro-Apple people, this was not particularly well-received) but, again, this will be addressed with future releases.
Also most unwelcome was the factory-set sensor life. Seven days without the possible of restarting is very surprising. There were some murmurings in the room about this setting a new precedent that other sensor makers would follow. Given that I am currently on day 18 of my sensor and the accuracy is spot on, I’d be bloody furious if I’d had to bin it 11 days ago!
Many of us frequently complain about the waste produced with all our device consumables, and there was some concern that the single-use sensor applicator contained a lot of plastic. Look, this is something that I personally struggle with. Every time I change my Dex sensor, or put in a new pump line or cartridge, I look at what needs to go in the bin and wince. It frustrates me each time I rip open the packaging for a new Medtronic Quickset (my preferred line), a bloody little cap falls out, usually to the floor. I have been using these sets since they first were released (maybe eight or ten years?) and never – not once – have I used the cap.
I get it – we need these consumables to be sterile. And safety and avoiding infection is paramount. But still, some of us are very concerned at the landfill we are contributing to!

Crowd sourcing opinion – What does CGM mean to you?
This discussion was very open. We were welcome to tweet, Instagram, Facebook (and blog) everything that we saw in the room, sharing it with the world. Following the demonstration, we all participated in real-time online feedback, where we commented on what we liked and disliked about the device. Our results and remarks were then shared on a screen for all to see.
Can we, for a moment, just consider how novel and out of character this is? Here is a company talking about a device that has not been released yet. And they are talking about it with a room full of over-sharers who all had screens open to various social media platforms ready to tweet, photograph and provide personal commentary. I have never seen such an open and transparent way to get feedback on a diabetes product, and the team from Roche should be absolutely commended on this approach. More please from more companies!

App making. (Photo credit: @Tadorna)
For the second half of the meeting we spent a most fun couple of hours where we played around with app development. My group – obviously the best – created an app that linked our CGM app with a juice machine to respond to low glucose levels. It also turned on bedroom lights if we were low overnight, to help wake us up. And if the wailing alarms of the app were not cancelled within 15 minutes, an ambulance was called to come and make sure we were okay. I know! Brilliant, right?!

Go team! Anna, Steffi, Sascha
Overall, this was definitely a valuable afternoon learning about new product and also being given the opportunity to meet with some very smart and active diabetes advocates. You bet we were there to be told about Roche’s new CGM, but that was only part of the event and no one in the room is so naïve they don’t know it. But the chance to share ideas and projects and plan for truly global work together outside the device company space was also achieved.
POSTSCRIPT and DISCLOSURES
I’m going to ignore the online discussions that seem to pop up at any conference where PWD manage to score an invite…. Actually, who am I kidding, I’m not. Because I am a little sick and tired of the inevitable complaining and suspicion and passive aggressive comments. I’m a huge advocate for PWD being invited to HCP conferences (I may have written about it once or twice here). For us to get here, we need financial assistance because travel is expensive as is conference registration. So when pharma or device companies offer to bring PWD together to engage in a session they are running – and also provide us with access to the conference, then you bet I am going to think it’s a great idea.
Transparency is important and on this little blog, I will always disclose any arrangements, support, funding or product in place with any company.
So…my disclosures? Well in regard to Roche, none really. I don’t use any Roche products at the moment. I have in the past used their meters, which I have funded myself. I have been an invited speaker at the Roche Educators Day at the ADS-ADEA conference two years running now. And I wrote and disclosed all about that at the time here and here.
Roche did not contribute to my travel or accommodation costs at all to attend EASD this year. They did provide me with press registration, but I had already organised my own, as I do for all conferences I attend. Oh – and they did invite me to a dinner after the blogger event, but jet lag had kicked in so I politely declined. There was no expectation from Roche that I would write about the event (or comment during it). They don’t own my words, I do. But I am incredibly grateful that they are engaging consumers in this way. So thank you to Ute and the team so very much!
As for my disclosures for attending EASD? For the third time, they are all here.
If you have not caught up with what happened in the opioid session at MedX this week, please do. ePatient, Britt Johnson, who blogs at Hurt Blogger wrote this outstanding piece about her experience on the panel in a session titled ‘The Opioid Crisis’ where she was pretty much ignored by the moderator of the panel. I read her piece in dismay because Britt’s experience is all too common.
As I wrote on Facebook yesterday, it is these twelve words of Britt’s post that are, for me, most telling:
‘The plan had been to feature me in the final five minutes.’
This was the response from the moderator of the panel when she was challenged as to why Britt has been ignored whilst on stage.
The final five minutes.
That’s right. After the healthcare professional experts got to say what they wanted; after the moderator directed the discussion in a direction to get what she wanted; after everyone but the patient was given an opportunity to speak. Then, and only then, would Britt have been given the opportunity to say what she wanted.
The final five minutes.
It’s the equivalent of being given a completed resource, almost ready to go to print and being asked to provide feedback. It’s the same as being a perfunctory consumer on an advisory board, often added at the last minute to tick a box.
This token and downright insulting attitude about where ‘patients’ fit into the healthcare puzzle is toxic.
We have become accustomed and too accepting of the status quo. We feel humbled when we are added to a panel discussion when, really, we should be the main event. We are honoured to be asked to provide feedback on an already developed service that we are meant to use because we mistakenly believe our opinion is being sought and matters even though it’s too late for our feedback to be taken on board. We believe we are doing well when a consumer is added to an advisory board made up predominantly of clinicians and researchers because, hey, it’s a start.
A start is not good enough anymore.
I am angry. I am so angry about this. I am angry about what happened to Britt at MedX. I am angry that there was not a single person with diabetes on the program at this year’s ADS ADEA conference. I am angry that it is 2016 and we still have to beg for a place at the table, on the panel, on the Board. I am angry that conversations ABOUT us are happening AROUND us. I am angry because there’s never a hesitation then it comes to convening a clinical advisory group, but a struggle with how and where to engage and appoint a consumer advisory group. I am angry because too many think ‘focus testing’ means engaging at the end, and that it is enough.
But mostly, I am angry at myself. I am angry for this post I wrote last year where I pathetically felt grateful because people with diabetes were quoted at a conference – not actually handed a microphone, not actually invited to sit on a panel, not given an opportunity to lead the discussion. We were quoted and I thought that was enough.
It was our final five minutes. And, actually, it wasn’t enough.
We are more than the final five minutes.
We are more than that; we are so, so very much more.
Apparently, I went to Munich. I was away for 6 days, and 60 hours of those days were spent in transit. I believe that, (as I deal with jet lag, hypoglycaemia, and mainlining caffeine), it is fair and accurate to say that I am too old for this shit.
I am also incapable of forming paragraphs. But dot points are fun! Here are some observations and a few silly thoughts from last week. (I’ll write some sensible things when my brain is back in the same country as the rest of me.)
- I am told by people Munich is a lovely city. I will have to take these people’s word for it, ‘cause I saw very little of the city.
- I did not buy a dirndl and for this, I will be eternally sorry. As will my husband.
- The EASD conference itself was, as predicted, very rats and mice-y. I sat in a number of sessions and wished I was a mouse (while wishing my diabetes away). Alas, I am not a mouse. And I still have diabetes. Damn.
- I did not get sick of laughing at the fact that one of the halls at ICM Messe München is called Langerhan Hall. Also, I did not get sick of saying ‘I wonder if my islet cells are in there’ – to everyone within earshot. Even if I didn’t know them.
- Obviously, Grumps was not as amused by this as me.

- As was the case at ADA, my arm is more recognisable and famous than me. A barista at one of the exhibition hall stands said, as making me a decent coffee, ‘Oh – I know you. I saw your arm the other day near the station.’ I am a walking billboard for Rockadex! (I am not sponsored by Rockadex and purchase my own patches.)
- Dr Kevin Lee from Queensland is a tweeting machine! In fact, I think the thing I was proudest of at EASD was seeing him tweet! (Actually, probably should say that Professor Mark Cooper’s giving the Claude Bernard Lecture was also a moment of national pride, but Kevin’s tweeting was on another level!)

- One of my favourite talks was about diabetes, cardiac health and exercise (go on, laugh….), but that was mostly because presenter Dr Nikolaus Marx, finished up with a discussion about passive exercise and cardiovascular events during the World Cup. If you were in the room, it was me who cheered when he mentioned the increase of cardiac events after Italy beat Germany. (#VivaItalia!). For clarification, I was cheering at Gli Azzuri’s victory, not the number of Germans having heart attacks. (By the way, this was a real study. Published here.)
- A HUGE shout out to these three women. AADE presidents elect, past and present, Nancy D’Hondt, Deb Greenwood and Hope Warsaw are absolute advocates for and champions of people with daibetes, and peer support. This is them at the docday blogger and advocate event. This level of commitment by HCPs to consumer engagement is enlightening, and a lot could be learnt from their example here. Thank you. Thank you. Thank you!

- It was wonderful to see quite a lot of discussion about AP. Anytime someone wants to actually get these into market and into the hands of people with diabetes, that would be just great! Thank you.
- I may have screamed a little when I wandered through the Association Village to the excellent historical display of diabetes by the German diabetes Association. Terrifying might be the word for these needles. I said a little prayer of thanks to the diabetes angels for modern day tech, and for being diagnosed in 1998.

- I need to take a break from twitter. This was the state of play when I said good bye to the conference.

And finally, a word about language, because I am unable to attend a conference and not talk and write about it. (I think it’s actually become a law somewhere.) This probably deserves a post of its own and that may happen, but here we go anyway.
This is the fifth EASD conference I have attended, and going in, I know that it is going to be challenging, language-wise. There often appears to be very little consideration that there could be PWD in the room listening in to how HCPs are speaking of us. In the opening ceremony, I tweeted this at EASD president, Professor Juleen Zierath:

I was a little disappointed at Professor Mark Cooper’s constant use of ‘diabetic’, but it was by no means any more than most of the other speakers. I suppose I just hold Australian speakers to higher account given the work we have been doing here around language and diabetes.
This tweet generated quite a lot of discussion, and came about after I was exhausted and annoyed and mostly frustrated by the way speakers were referring to people with diabetes.
 Perhaps the best response was from Nick Oliver:
Perhaps the best response was from Nick Oliver:

Here’s the thing – and it is something I spoke about during my talk at the DOCDAY event. Language DOES matter. We all know that. It’s completely and utterly disingenuous to say it doesn’t. For some people, it doesn’t bother them and that’s terrific. For others, though, it really does. So why would anyone do something that may offend when it is so easy to avoid that?
DISCLOSE DISCLOSE DISCLOSE
My (economy fare) flights and accommodation expenses were covered by AMSL and J&J. I was attending the EASD conference mainly to attend the J&J DOC exchange meeting which I was involved in preparing and presenting. No one ever expects me to write anything. These are my words and observations only. (And seriously, have you seen what I have just written? No one wants to be associated with that!)
 I’m in Sydney for the next couple of days for the Diabetes Exchange (DX) program hosted by Abbott Diabetes Care. The event which runs over two days is part of Abbott’s global DX initiative which aims to bring together diabetes bloggers from all over the world. The Sydney event follows on from the initial meeting in Berlin, and next month, there will be an EU event, this time in Stockholm. (How’s your French? Google translate may be able to help you with this wrap up of the Berlin event from my dear friend Andrea.)
I’m in Sydney for the next couple of days for the Diabetes Exchange (DX) program hosted by Abbott Diabetes Care. The event which runs over two days is part of Abbott’s global DX initiative which aims to bring together diabetes bloggers from all over the world. The Sydney event follows on from the initial meeting in Berlin, and next month, there will be an EU event, this time in Stockholm. (How’s your French? Google translate may be able to help you with this wrap up of the Berlin event from my dear friend Andrea.)
I am terribly excited about the Sydney event for a few reasons. Firstly, it’s all about the tech. Make no mistake, we’re here to talk about the Abbott Freestyle Libre monitoring device which (finally) received TGA approval earlier this year and will be launched into the Aussie market in the very near future. It’s exciting technology – and how clever are Abbott in bringing together a group of bloggers who may just share their experience (of both the event and the device) with others?
I am also excited because I will be surrounded by my peers – others who also live with diabetes. I will be with old friends, others that I only know in the 140-characters-or-fewer world of Twitter and others that I have never met (in real like or online) before. I am especially excited that it is an Australian event because it is always fascinating to see and hear the close-to-home perspective and just how different it can be even though we are accessing the same health system while living with same health condition. (I’m also thrilled to be in a room full of Aussies because it means I won’t spend a significant part of the day asking ‘Does that translate?’ as I try to explain something that makes absolutely no sense to anyone from Europe, the US or the UK.)
I have been very fortunate to sit around tables around the world at similar events and the power and value is not because we all agree with each other. In fact, the real magic happens when there is respectful and robust discussion where everyone is given the opportunity to safely share their experience and perspective. I so hope that is the outcome of the DX2Sydney meeting.
I am facilitating DX2Sydney, which is terrific because it means I don’t have to say much. I mainly get to listen and hear others’ thoughts. The agenda is packed full of interesting topics and there is plenty of time for tangents and questions. Well done to Abbott for bringing us all together. This is a terrific opportunity for us as bloggers, but it is also such a brilliant opportunity for Abbott to get some valuable insight into working with PWD. We have a place at this table; there is no issue with industry and PWD talking, engaging and sharing. I’m so pleased to be here to do that!
Our Aussie blogging community may be small – and this may be only the second time that a group of Aussie diabetes social media influencers have been allowed in a room at the same time (the first time being Diabetes Victoria’s innovative Diabetes SoMe Summit back in 2012), but I am so pleased that we are being given the opportunity to lend our voice to the global DX initiative.
You can follow along on Twitter at #DX2Sydney. And I’ll be linking to any blogs or commentary from the other attendees in later posts.
DISCLOSURE
DX2Sydney is being coordinated and run by Abbott Diabetes Care. The costs for me to attend the two day event (travel, accommodation, meals and transfers) have been covered by Abbott. All attendees will also receive Freestyle Libre product so we can trial the new device.
There is no expectation that I will write about the event or my thoughts of the device. Abbott may have paid for me to attend, but they have not paid for my words on this blog, social media activity or anywhere else. I do, however, promise to try to keep myself nice and not swear. (But that could go pear-shaped any moment!)

DX2Sydney is being held in the ACDC suite of the hotel. Who said diabetes isn’t rock ‘n’ roll?
I am all for consumer engagement. ‘Ask people what they need,’ I say all the time. ‘Give the people want they want!’ I implore audiences at conferences. ‘By consulting, you get buy in,’ I promise in meetings.
And then, what happens? People are asked. There is a public consultation. There is buy in. And the result?
This. This is the result.

This is why we can’t have nice things!
Thanks to Melissa Lee for posting the link on Facebook earlier today. I was laughing so hard I had to close my office door to hide the tears rolling down my cheeks.
P.S. Regardless, I am still a huge advocate for consumer engagement.
P.P.S. My vote is actually for RRS Pingu.









